

GLAZES: FROM ASH AND CLAY
by Stan Prokop
Last summer I decided that I would specialise in developing ash glazes in my pottery classes. I was inspired by Phil Rogers' book "Ash Glazes" and began to understand why I liked the sort of pots I liked. The ash glaze potter throws and turns a form in such a way as to complement his sense of how the glaze will collect and flow; how grooves here, or gutters there, or a step will form a darker line, an accent or start a series of runs. Such glazes emphasise a shoulder, or belly, or rim, separately or together. They can emphasise a vertical or a horizontal to complement or contrast the visual rhythms of a piece. The rhythms are often minimalist: the colours muted and spare, their harmonies coming from subtle gradations of tone within the same colour, often. Contemporary potters can turn all of this on its head, but their power is in part derived from an echo of what the modern piece is not.
After lots of bonfires in the autumn I had collected a meagre sample of mixed ash and developed some method of making line blends and weighing very small amounts of ingredients accurately. I was ready to start.
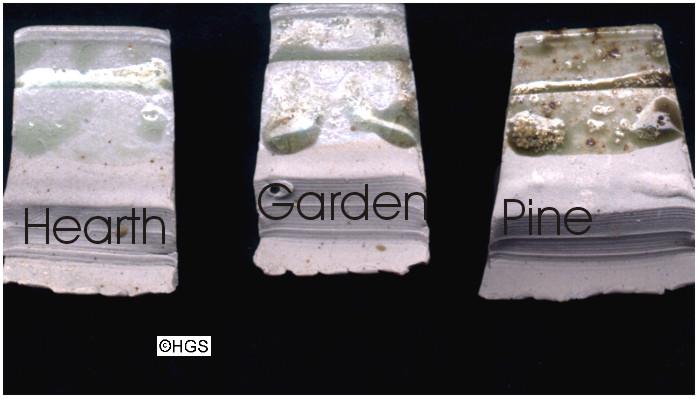
The first illustration shows the sort of glaze pure ashes make. The Hearth sample was from a winter's burning of mixed, primarily hardwood logs. The ash which must often have been re-heated to high temperatures, was dense and white. It shows the grey green colour resulting from a low iron content (about 1%) and it virtually makes a glaze of its own. It is likely to be similar to the first accidental glazes fired by the new kiln technology of China some 2,000 years ago, when the ashes of the wood used to heat the kilns blew over and settled on the stoneware pots. The garden sample was from an ordinary bonfire with mixed wood and garden debris. It is darker, has more "bits" (unsieved) and shows the higher iron content (2-3%). Last is a pure sample of pinewood ash, which is well known for having the highest iron content of all the wood ashes and regularly makes a bottle green glaze (4%+). I hope that they demonstrate a baseline from which to make sense of the more complex glazes.
I thought it would be in the spirit of authenticity to find some local clay (I live in Sutton, south London) and I gathered two samples from the bank of a local river, an ochre and a grey. In addition to each standard glaze, I added 2 and 4% of iron in the forms of yellow ochre (heavy grade) and red iron oxide. I used a long standing standard recipe of 40 parts ash, 40 parts feldspar and 20 parts clay. My tutor, very helpfully, brought in some of his dry clay samples and I was able to do a first run. The series of glazes were painted on to white clay standing tiles and fired in a reduction gas kiln. The results were surprisingly (to me) rich and textured. I saw that the standard commercial china and ball clays I had used in all my development tests before, were so refined, or naturally deficient in impurities, that they produced a smooth result only.
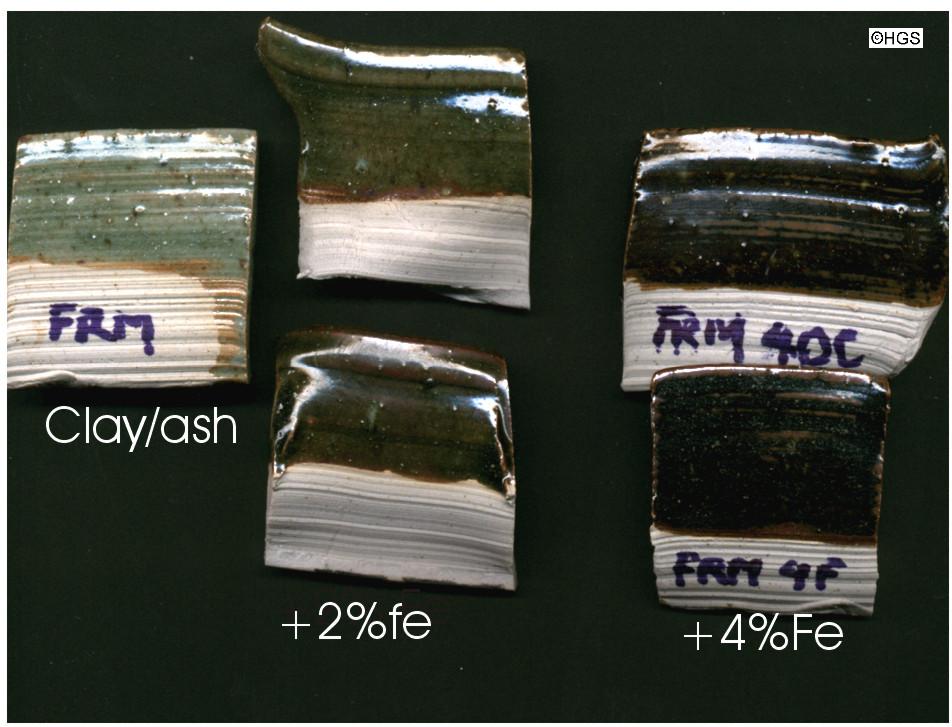
The second illustration shows one of that series derived from Fremington Clay. The pure glaze is richer, with a light grey green and a tinge of yellow. The speckles are probably derived from iron and/or manganese in both the ash and the clay. The series shows how the addition of iron to the glaze moves it into an olive green celadon colour (2% added, probably 4% total including the iron in the ash and the clay). The addition of 4% iron turns it into a dark brown to black tenmoku glaze colour.
The focus of my interest suddenly changed. I realised that the most insignificant ingredient of an ash glaze could have a profound influence on the result and that the clay fraction was worthy of independent study.
What did I know about clay? What about geology? Didn't it all come in plastic bags? Do not envy my journey from that naive ignorance to the horrible complex truth. Potters, on the whole, may dig up the local countryside or order plastic bags from reputable firms who work hard for reproducible results. They have their own descriptive vocabulary and literature. Geologists work over much greater geographical and temporal scopes, have a dense specialist vocabulary and produce intense and confusing maps and pamphlets hidden away in the Natural History Museum. They are guarded by giant prehistoric monsters who may just come to life. Some of them are secretive and probably rich and feel you are seeking out their hidden treasures of gold and oil. They never answer any e-mails. It was beginning to feel like the Lord of the Rings all over again. Clay chemists seem to live in the blinding white light of a new age; the high priests with the purest of rays and samples. They are an international band whose monographs could collect spells from Minnesota to Yugoslavia, from Russia to Japan. Chromium and titanium, manganese and magnesium could ooze from their interstratification layers at the drop of a hat. Did they talk to each other? I'm still not sure... but Drs. West and Clayton from Southampton, Dr. Adrian Lloyd-Lawrence from the Mineralogical Society and Mike Horne from Hull talked to me and allowed me to move through their different worlds. I began to link the geological map to the cliff face and the coastal exposure: to link a period in the earth's development with a different geography and the elements the clays carry and fuse at 1280 degrees C. to form a coloured glaze.
Mike Horne was kind enough to agree to send some samples of clays and silts from his forays in search of micro-fossils. I am deeply indebted to him, not only for sending me some 14 different samples, but for linking them to the geological time-line. Locally, although the winter floods had eroded the river bank, a waterboard was renewing 4 miles of water pipes up hill and down dale through various layers of the London Clay, judging by the different visual appearances. The Claygate Member of this series is named from a nearby village where a redevelopment had bored 5 metres (15 feet) and I was encouraged to collect the "all sorts of colours " the builder had excavated. Three potting friends were persuaded to excavate areas of Frome, Yarmouth (Isle Of Wight) and Freshwater Bay (Isle Of Wight), although they thought my maps of the cliff exposures were going too far! These clay samples, washed, sieved and dried provided a series of 43 glaze tests.
Here, I will concentrate on the East Yorkshire samples. They are shown in illustration 3.

All the samples were constructed at the same time and were fired together. The first "ball clay glaze" was typical of the smooth pure glaze derived from commercial preparations. It was dug from Frome. The second, derived from London Clay, is darker and contains some speckles, which can become very pronounced in some strata which are pyritic. They both provide useful comparisons:-
What then are the main colour differentiators between these clays? Iron and Manganese mostly! The iron content is the most important. It is lowest in the kaolins and ball clays of the south-west. Electro-magnets purify the little there is. The halloysites can contain 13% iron (Japan) and chromium at 12% (Yugoslavia and California) derived from hydrothermal clays, but this is rare. The tri-octohedral serpentines can be rich in iron, manganese (reddy, rather than yellowy-brown) and nickel (greying). Non-planar serpentines can have iron levels in the 50% range and others, Manganese levels in the high 40%. Talcs can contain nickel levels as high as 34% and iron in the 25% range. The dioctohedral smectites carry iron "in the pottery range"; a montmorillonite from Redhill in Surrey having 8.25%. Trioctohedral smectites can contain iron, lithium, chromium, copper, zinc, nickel and vanadium. These are all colouring oxides relevant to glazes. Vermiculites can have high iron content and up to 2% titanium. The mica clay minerals (illite) can have strontium, barium, rubidium and caesium in their interlayer sites and have unusual compositions to include chromium, vanadium, manganese, nickel, cobalt, copper, barium, beryllium and boron. Within this group the glauconites and celadonites {please check this word} have iron in the range 17-21%. Tri-octohedral mica can also have considerable titanium content. I have found little in the literature, so far, that gives a detailed picture of chromophoric compounds in British clays, but it may be worth pursuing the quarrying company chemists. I have been encouraged by my journey so far. My next journey may be into the Norfolk clays, for example, which provide an opalescent blue, of all things (probably high in boron). If you have any potting friends, give them a surprise muddy present! You never know what you or they will find.
Bibliography:
Michael Cardew 2002. Pioneer Pottery. Ceramic Classics, A & C Black, London.
A.C.D.Newman (Ed.) Chemistry of Clay and Clay Minerals (Mineralogical Society Monograph no.6).
J.M. Ridgway 1982. Common Clay and Shale. HMSO, London.
Phil Rogers 2003. Ash Glazes (2nd Edn). A & C Black.
A.B.Searle 1912. British Clays Shales and Sands. Charles Griffin.
Brian Sutherland, 1987. Glazes from Natural Sources. B. T. Batsford.
Contact details for author - 16 Camborne Road, Sutton, Surrey, SM2 6RH; 'phone 020-8642-4602.
July 2004
copyright Hull Geological Society 2020