

Humberside Geologist no 10
published 1992
Artists impression of a flint and radiolaria against a fictitious chalk section.
What is the origin of flint in chalk?
And why does it occur in only a part of a sequence?
This forms a very interesting study and it is natural that many of our readers have a deep interest and have become involved.
Chert and flint are the two most common siliceous sediments. They can be classified under the Silica Group of minerals along with quartz, chalcedony, opal, agate and onyx. Chemically it is SiO2.
Chert and flint can be composed of one or several forms of silica. Usually it is dull grey to black in colour. Its hardness is about 6 1/2 on the Mohs' Scale and when dropped will break with a pronounced conchoidal fracture. When broken it gives sharp edges a property exploited by early man in making tools. The term flint is widely used as a synonym for chert and describes the black nodular variety which is commonly found in chalk. Chert is widely distributed and can be found in carbonate rocks of all ages, while flint nodules are characteristic of Cretaceous chalk of both England and France.
The origin of flint has baffled geologists for some while. But gradually the puzzle was refined to a choice of two theories:
1) That the silica is deposited on the sea floor contemporaneously with the chalk, and
2) That the silica is a post-depositional replacement of the host rock by silica from percolating waters.
There were adherents of both concepts who argued stoutly for what they believed to be correct.
In favour of (1) some workers considered that the flints formed by direct precipitation of masses of silica gel on the sea bed.
The argument against (1) is based upon geochemistry and makes it appear unlikely. In simple terms, the reason is that sea water contains only about 4 parts per million of silica. At a temperature of about 25°C silica can be soluble in sea water up to around 140 parts per million.
It is known that siliceous sponges, diatoms, and radiolaria extract silica from sea water to form their hard parts and so keep silica concentration low in sea water. And so one can argue that flint does not precipitate in a normal marine environment. Moreover, it appears that nobody has identified a site where it is happening today.
So were the seas of a different composition than they are today? The difference would have to be by a factor of 35 and that sounds improbable.
Those in favour of (2), that flint is post-depositional and is a replacement of the host rock invoke an epigenetic process: it implies a change in the mineral character of the rock owing to "outside" influences. By this is meant influences surrounding and outside of the flint itself.
And what might these influences be? Of course they would have to be a change in the chemical environment within the chalk itself. What better mechanism is there than the circulating pore waters.
So we now have two more technical terms to consider: Is the formation of flint an authigenic or a diagenetic process?
Authigenesis is the term which describes chemical reactions at the sea water/sediment interface.
Diagenesis is the term which describes chemical reactions that occur within the sediment.
But researchers find it difficult to draw a hard and fast line between the two processes since authigenesis continues after burial of the newly deposited sediment. So can we be sure which is which?
Because of this uncertainty, you may find statements in geology textbooks which may appear contradictory. Again, one may read of the search for flints which display the features of a bedding plane in order to prove theory (2). But the fact that not all flints exhibit this feature does not mean that some can be regarded as evidence for theory (1). A researcher in favour of theory (1) may argue that the sea water/sediment interface has experienced a change in the chemical environment.
Others may point to the fact that flints can form as nodules and also as tabular beds extending great distances. But when one sees flint forming along a near vertical fault one naturally favours theory (2). Also the fact that the lithification of calcium carbonate sediments to form chalk or limestone is a gradual and long-term process encourages one to go for theory (2). The formation of chalk and limestone is safely described as diagenetic.
What might these changes be within a rock or sediment? There is temperature and pressure, salinity changes. But there are other important changes: the change from oxidizing to reducing conditions as well as a change from acidity to alkalinity in the environment.
These last two items can be expressed in what are called Eh/pH diagrams and these diagrams are often found in books on geochemistry to explain why certain minerals are deposited under known conditions.
An Eh/pH diagram may be used to illustrate how the changes in environment can promote or prevent the formation of minerals such as silica and calcite.
Fig 1 is a typical Eh/pH diagram and shows how these two influences combine to produce environments which are suitable for the deposition of minerals according to the reducing/oxidation potential (Eh) and the acidity/alkalinity potential (pH)
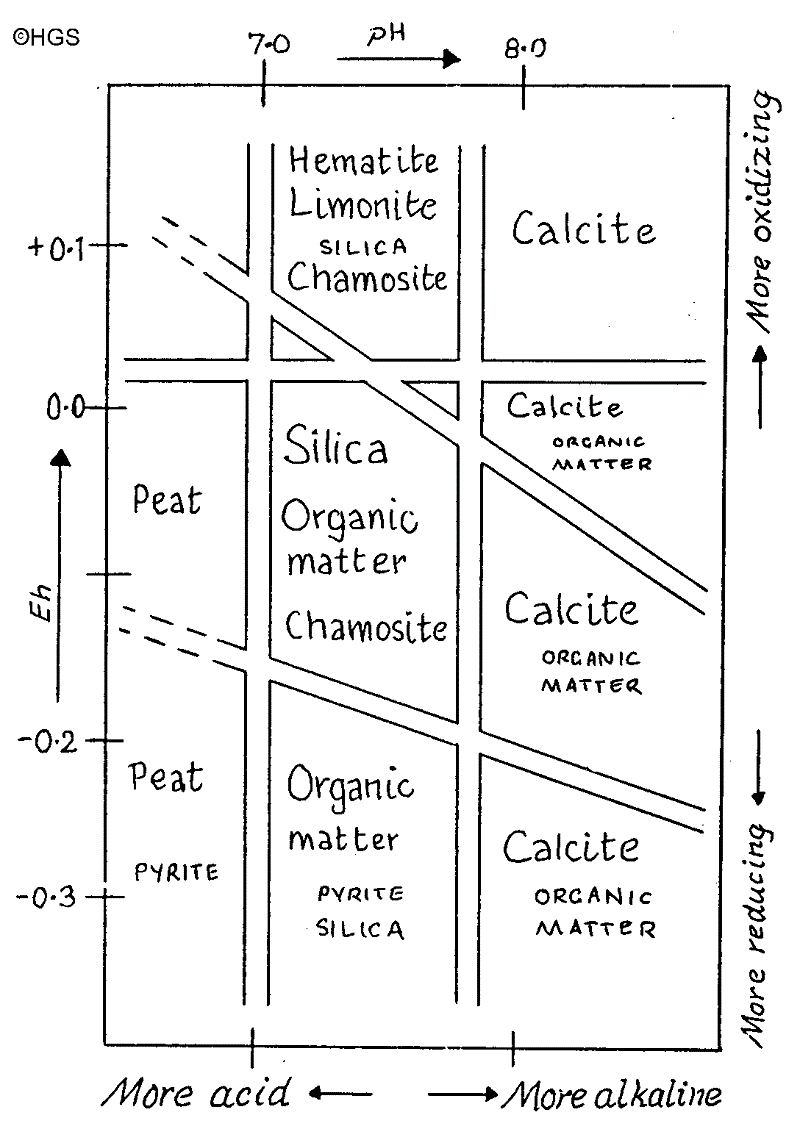
Fig 1 This is a Eh/pH diagram which shows a variety of environments which allow different kinds of deposition or formation. Note that it is conventional to plot the Eh on the vertical axis and the pH on the horizontal axis.
It shows that silica is best precipitated in the region of pH 7.0 to 7.8 with Eh zero to -0.15 approximately. And by contrast, calcite is precipitated above pH 7.8 and over a wide range of Eh in the diagram. So the environment needed for the deposition of chalk (calcite) is different from the environment needed for the formation of flint (silica).
So calcite would dissolve while silica precipitates. Here we have the geochemical basis for theory (2). We also have another contrast: while sea water everywhere is undersaturated with respect to silica, only in deep waters is CaC03 (calcite) undersaturated.
Modern research in oceanography has given us the knowledge that as many as 80 chemical elements exist in present—day sea water. And of these no less than 43 occur in the hydrated form, as follows:
Silica in the form of Si(OH)4
Iron in the form of Fe(0H)2 and Fe(OH)3
Samarium in the form of Sm(OH)3
Ytterbium in the form of Yb(OH)3
and so on…
We now know that there is a wide variety of suspended particles in sea water. Some is in solution while some is called particulate matter since these materials tend to coalesce to form colloidal particles. It has been shown that if the tiny hard parts of dead radiolaria sink gradually to the sea floor they will become dissolved. Gravity alone will not bring them to the sea bed. But siliceous material in the form of faecal pellets or accumulations in the form of marine "snow" can and does reach the sediment/sea water interface.
Siliceous débris must fall very quickly since only about one to ten percent escapes dissolution on the way down. The larger the supply of siliceous skeletal material the more likely some will reach the sediment/sea floor interface. And one more point: this could be quickly swamped by any high input of terrigenous sediment transported to the sea by rivers in flood.
Recent work by Clayton supports theory (2). He believes in early diagenesis at a level of about five to ten metres below the sediment/sea water interface. It is suggested that a porosity of about 75-80% would allow the precipitation of silica and the dissolution of the host rock. The upper layers of the chalk were progressively depleted of dissolved oxygen in the pore waters by aerobic bacterial degradation of organic matter.
H2S is released by sulphate reduction, and this mixes with the oxygen (O2) which travels down with the porewaters. This mixing takes place in the reduction/oxidation zone (the oxic—anoxic boundary where the flint is formed). It is thought that the precipitation of flint was triggered by the dissolution of the host rock as the Eh/pH environment changed in its favour.
It is thought that reduced permeability of pore waters produce tabular flints while a greater permeability variation of upward diffusing sulphide and downward moving 02 would be a suitable environment for the formation of nodules.
Modern studies in oceanography show that today the uppermost few millimetres of sediment below the sediment/sea water interface tend to become more saturated with both carbonate and silica. Although this will fluctuate with the flux of siliceous débris, undersaturation can be restored by exchange with bottom waters. But the Cretaceous were times of great productivity with very high sea-levels. Some workers suggest the seas were about 450m above the present level.
This high sea-level would mean a transgression of the shallow continental areas and the formation of what is known as epeiric seas.
These epeiric seas developed along eastern and south-east England as well as in France and many other parts of the world. The Chalk Seas of NW Europe are a product of the special conditions existing in the Cretaceous. In these new shallow seas productivity of calcareous marine phytoplankton was abundant and provided material for the thick beds of chalk. Likewise the productivity of the siliceous plankton increased. During this period there was no Ice Cap so no cold bottom waters flowed into these Chalk Seas.
The massive output of carbonate from these seas continued to close on the Cretaceous/Tertiary boundary (often known as the C/T boundary or K/T boundary by cartographers). Afterwards there was a rapid lowering of sea-levels and a wholesale extinction of calcareous organisms. And the Chalk Seas came to an end as well.
Selected Bibliography
Clayton, C.J. The Scientific Study of Flint and Chert Vol 1, Cambridge UP, pp 43-55 (1986)
Bearman, G. (Ed) Ocean Chemistry and Deep-Sea Sediments, Pergamon (1989)
Kennett, J. Marine Geology (1982)
Pettijohn, E.J. Sedimentary Rocks (1975)
Stowe, K. Ocean Science, Wiley (1983)
Wood, C.J. & Smith, E.G. Proc Yorks Geol Soc 42 pp 263-287 (1978)
Wooley, Hamilton, Bishop, Minerals, Rocks and Fossils (1974)
How often do the people of East Yorkshire ponder the fact that they live on the eroded sea bed of an ancient epeiric sea ?
Often this epeiric sea is known as the Cretaceous Chalk Sea of North Western Europe. But what exactly is an epeiric sea?
An epeiric sea is formed when sea water rises and floods over considerable areas of low altitude continental crust which normally (that is today) is above the sea level. The term continental crust is used deliberately to contrast with ocean crust which is basaltic and is normally the sea bed.
Before the plate tectonic revolution it was usual to speak of "sial" and "sima", but today is has become more fashionable to speak of ocean crust and continental crust respectively. These terms are considered to be more meaningful today in the light of modern plate tectonic theory.
For many years it has been known that an Ice Age will lower the world's sea level because of the simple reason much of the water has been used up to form ice. But, outside of an Ice Age there was little comprehension of any cause for sea-level changes. That is until the concept of plate tectonics was pondered more deeply and developed.
Much of this modern research was done by Hays and Pitman and later by Donovan and Jones.
Since the amount of sea water appears to have remained fairly constant, we need to look to changes in volume of ocean basins for the answer.
Before we go any further we need to recap on the basic terms of plate tectonics: A Mid Ocean Ridge exists in most oceans which is active volcanically. It is also known as a spreading ridge because it erupts basaltic lavas. In short hand one usually refers to it as MOR; and the rock which it produces is MORB or Mid Ocean Ridge Basalt. At a MOR the eruption of basalt produces new ocean crust called OC for short and the two sides of the MOR move apart at a rate of about 2cm per year causing continental drift.
An ocean with a MOR is obviously an expanding ocean; but if it also has a subduction zone it can become a contracting ocean.
Well, we are looking for a reason for a change in volume of an ocean basin. For starters, if we ponder a world tectonic map illustrating the ages of the ocean floor we will find that production was not constant but more was produced at certain times than others.
This indicates changes in degree of volcanic vigour at the MORs. At times of increased volcanic vigour, more basalt is erupted at these MORs. More MORB is formed. Increased amounts of OC is formed per year.
Now, the MOR becomes heated with the molten rock in the magma chamber below. At times of increased vigour there will be more heat and so the ridge becomes more hot. Hot rock expands, becomes less dense therefore, and the height of the ridge increases. The importance of vertical movements was recognized by Hallam (1963) and Menard (1964). They calculated the volume of the MORs world-wide as some
1.6 x 108 km3
and against this we need to compare the total volume of sea water as some
1.35 x 109 km3
I think you will agree that these figures are significant. From this it follows that even small changes in the height of MORs will make significant changes in their volume and in the volume of ocean basins.
In addition, the volume of ocean basins will change according to the variation in the total length of the world's MORs.
This may seem a surprise at first. But one has to remember that the geography of this planet has never been static. Even during our lifetimes, we must be aware of places around our own coastline where there is a net erosion of the coastline or a net gain. In addition to the constant erosion and deposition which takes place around our coasts there are also other factors which can often be forgotten:
They are RIFTING and MOUNTAIN BUILDING.
Maybe these two activities are not taking place in East Yorkshire today! But to understand the reason for the Chalk Seas of the Cretaceous we should not overlook the aspects of rifting and mountain building in our consideration of the changes in ocean volume.
During times of more active rifting we can expect the volume of the ocean basins to become smaller while during times of increased mountain building we can expect the volume of ocean basins to be enlarged! During Cretaceous times both the total length of the MORs as well as their volume increased. The volcanism at the ridges become more vigorous.
As a result of these changes the world-wide sea-level increased by between 300m to 350m. And this meant that new shallow seas were created over large areas of low altitude land. The Chalk Seas of North West Europe were among these newly created epeiric seas which extended over large areas of Europe and other continents.
You can imagine the effect this would have. Wise (1974) estimated that some 60% of the continental crust was flooded during the peak time in the Upper Cretaceous.
The effect of increasing the area covered by sea water was to reduce the thermal gradient toward the poles. This warming and mellowing of the climate naturally provided the décor for the warm, shallow Chalk Seas which we label epeiric seas.
Fig 2 explains graphically the dramatic extent of the transgression of the sea during the Cretaceous compared with the Jurassic and the Tertiary. Note how the transgressions are characteristically gradual while the regressions are rapid.
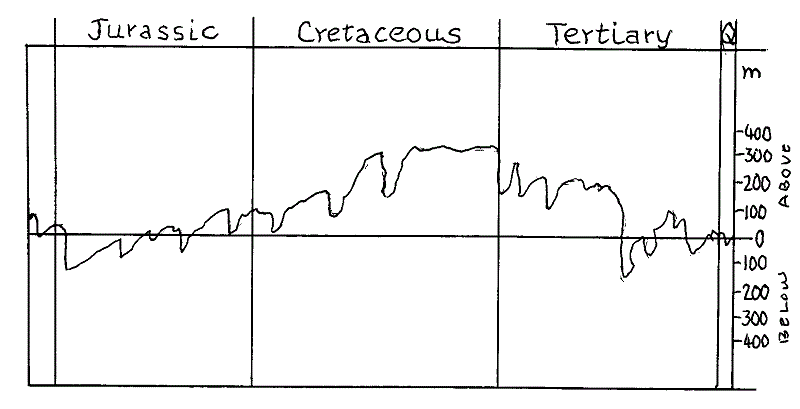
Fig 2 World wide sea-level changes during the Jurassic, Cretaceous, Tertiary and Quaternary. The scale shows 0 as the present day sea-level with metres above and below. It shows that the Upper Cretaceous was a period of environmental stability followed by a very rapid change due to the sudden regression.
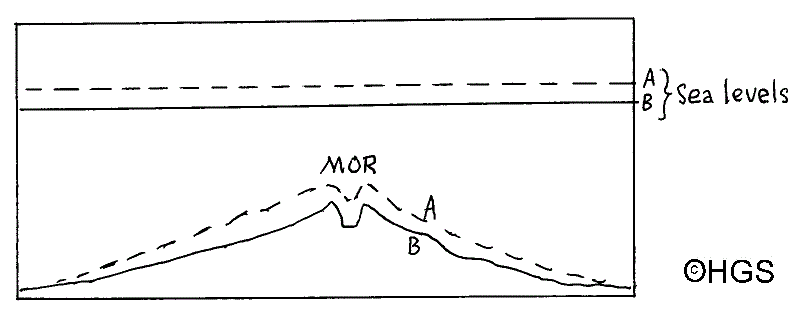
Fig 3 During times of increased volcanic activity at a MOR when the spreading rate is greater the ridge will be higher (A) compared with (B) when volcanic activity is less vigorous.
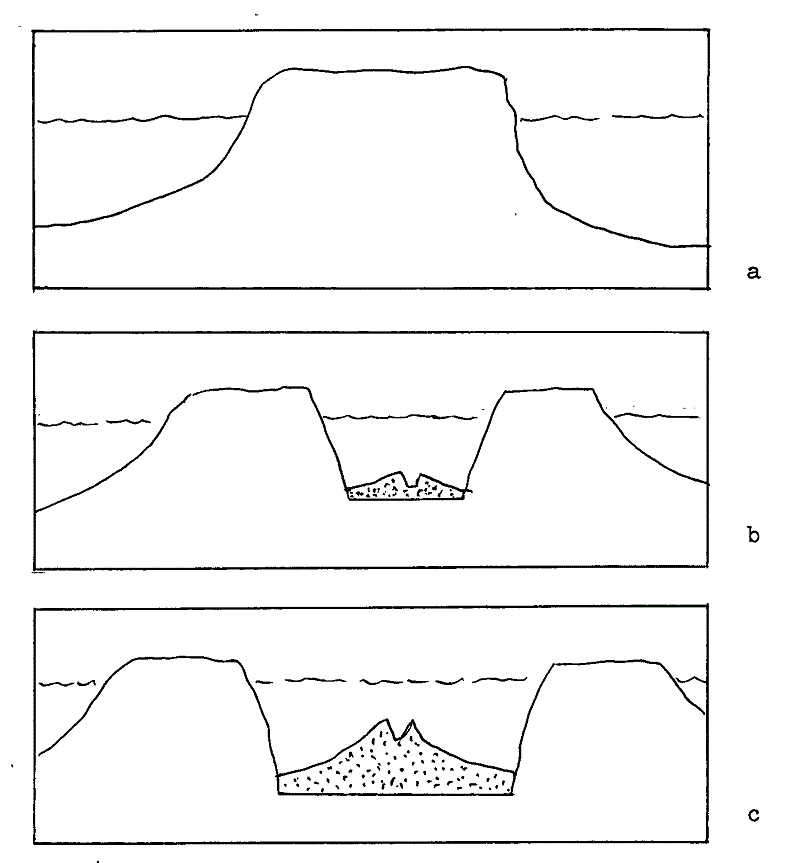
fig 4 (a) shows sketch diagram of continent with sea-level, (b) shows the onset of rifting of the continent with a new MOR (stippled), and (c) shows successful continuation of rifting with volumetric increase in MOR and creation of a widening ocean. A sea-level rise has taken place between (a) and (c).
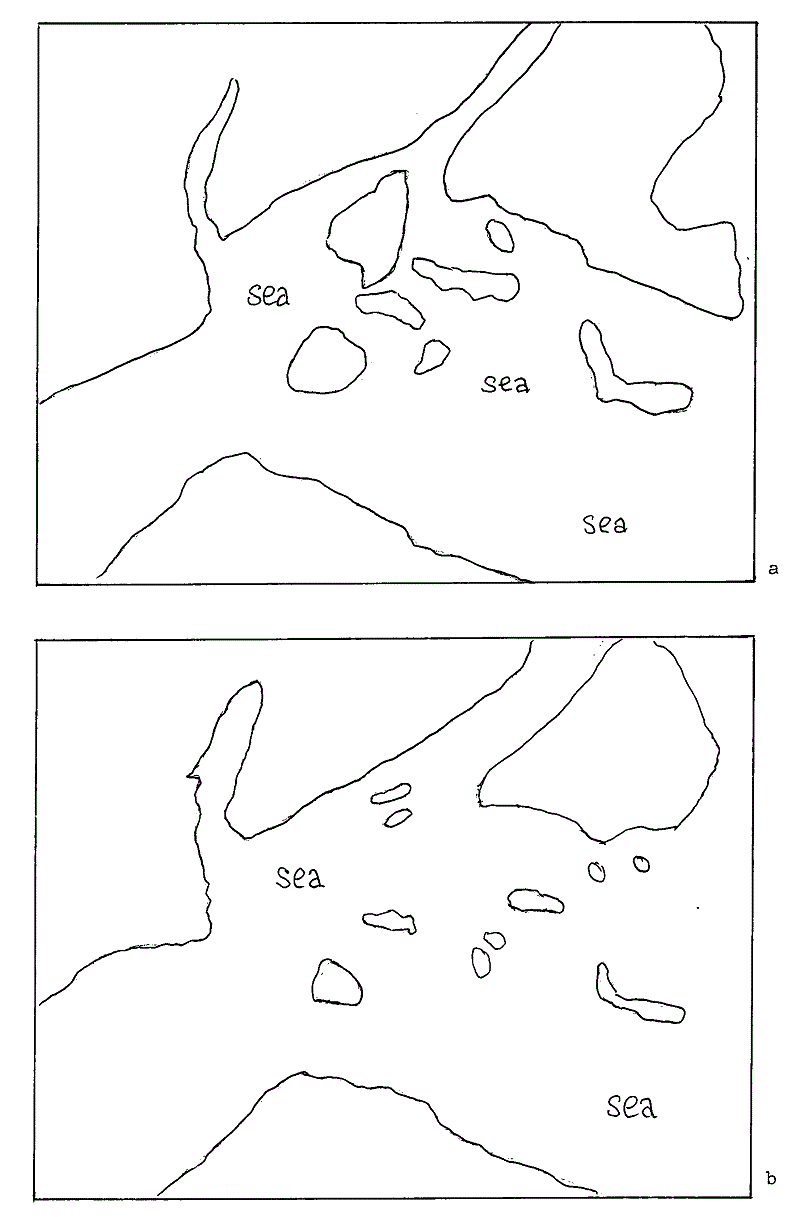
Fig 5 Sketch maps showing approximate land areas in the Lower Cretaceous (a), and during the Upper Cretaceous (b). You are invited to take an A4 sheet of tracing paper and to trace both (a) and (b) and to lay your sheet upon Fig 6 on the next page to obtain an indication of the geography relative to the coastlines we understand.
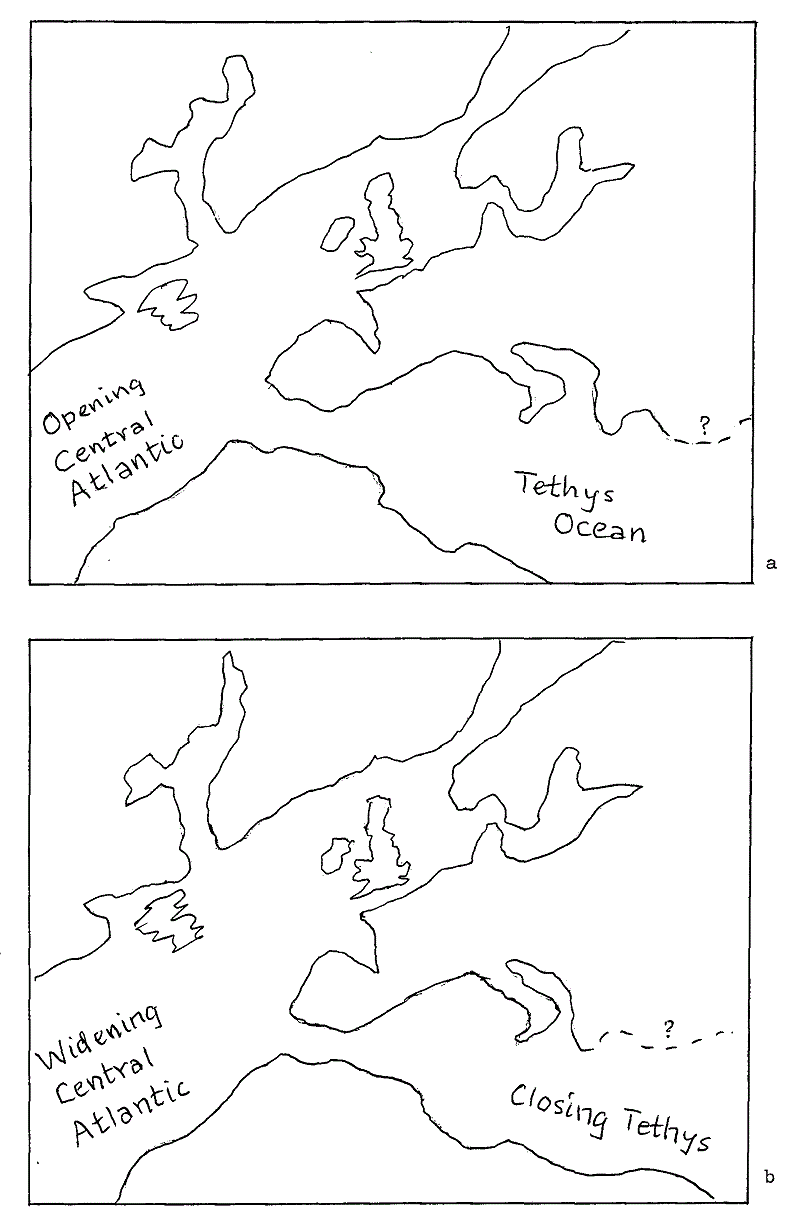
Fig 6 Sketch base maps showing Europe during the Lower Cretaceous (a), and the Upper Cretaceous (b). These have been adjusted for continental drift. Lay your trace of Fig 5 over Fig 6 and you will get an impression of the extent of the epeiric seas of Cretaceous times, part of which is the Chalk Sea of north west Europe which extended over East Yorkshire.
Fig 3 and Fig 4 illustrate how increased volcanic vigour at the MORs reduce the volume of basins in oceans by increasing the volume of the ridges. To this we must add the extra MORs which were created during that phase of the break-up of Pangaea. Fig 5 and Fig 6, when used together, show graphically the extent of the epeiric seas of the Upper Cretaceous. The uniform, warm, and stable climatic conditions reduced the temperature gradient toward the poles and allowed significant increases in faunal diversity. Two fine articles appeared in Humberside Geologist describing the faunal richness of the times by Latham & Whitham, and by Fenton so there is no need to discuss this again here.
It is clear that wide adaptation to the niches in these stable conditions must have produced inherent vulnerability to any change. And a major change did come after about 70Ma when the epeiric seas regressed. It has been speculated that land areas probably doubled by the time of the C/T boundary. This produced the opposite effect from transgression when the epeiric seas were created, namely, a more continental type of environment with more seasonal contrasts, and increased thermal gradients toward the poles. These will have produced enormous stresses upon species adapted to the more stable times of the Upper Cretaceous. And not only were the Chalk Seas destroyed with their rich faunal populations, but the dinosaurs could not cope either!
Bibliography
Donovan, D.T. & Jones, E.J.W. J Geol Soc London, 136, (1979)
Fenton, K. Intro to the Study of the Chalk, Humberside Geologist Number 6 (1988)
Greenwood, P.H. (Ed.) The Evolving Earth, (1981)
Hays, J.D. & Pitman, W.C. Nature, 277, (Nov 2) (1973)
Latham, B. & Whitham, F. Belemnites from the Cenomanian of Melton Bottoms, Humberside Geologist Number 5 (1986)
Pitman, W.C. Geol Soc Amer Bull, 89, pp1389-1403 (1978)
Smith, A.G. & Briden, J.C. Mesozoic & Cenozoic Paleocontinental Maps (1977)
Wise, W.C. The Geology of Continental Margins, NY (1974)
Now we are armed with some of the present thinking on the subject, it will be enjoyable to have a brief glance at some selected excerpts from earlier geology textbooks.
There is a tremendous amount of literature in the subject of chert and flint and checking through a large number of books on the shelves of a consultant friend I have been able to select some of the choicest of references. They indicate that the subject of the origin of flints never was an easy one. Yet it is interesting that many authors glimpsed some of the truths. Even so, progress was slow. For example, Hatch, Rastall and Black writing their book entitled "Petrology of the Sedimentary Rocks" in 1936 stated that papers by Sollas in 1880 and 1887 on the subject of flints may be taken as presenting the accepted understanding of the thirties.
In 1926 Twenhofel wrote:
Chert and flint occur in the form of globular, ellipsoidal, discoidal, and irregularly shaped nodules or concretions; as lenses; beds; cavity fillings; and cement for other types of sediments. Vein flints and cherts also occur .....
In 1944 Holmes wrote about flints and mentioned some valid points such as "whenever conditions were favourable" but did not know what were the favourable conditions. He also thinks the flint was formed after the uplift of the chalk but does not say why he holds to this theory. He writes:
Ground-waters percolating through chalk at some stage after its uplift from the sea floor picked up colloidal silica from minute and easily dissolved opaline sponge spicules dispersed through the formation. By replacement of calcium carbonate, wherever conditions were favourable, the silica was deposited as flint which, being insoluble, was not redissolved again.
The return of dissolved material to the rocks through which ground water is circulating involves the operation of much more complex and delicately balanced processes which are still but little understood.
Precipitation may be brought about by such factors as loss of gasses and consequent decrease of solvent power; cooling while waters are ascending; changes of pressure during circulation; or the mingling of waters of different sources.
In 1954 Goldschmidt speaks of flint as an epigenic product and describes flint as the most spectacular example of silica in layers and veins. He also sees it as an epigenic product but connects it with surface solutions:
The precipitation of amorphous opaline of chalcedonic silica from surface solutions in non-volcanic districts is a phenomen well known to geologists, although it has not been sufficiently studied.
Precipitation of dissolved soil silica from weathering processes is well known in the arid or semi-arid districts of lower latitudes, for instance, in South West Africa. But the most spectacular example of precipitation of dissolved silica in geological times is the formation of the "flint" concretions, nodules, veins, and layers in the calcium carbonate muds of the Cretaceous formation of North West Europe, especially in Britain and northern France.
Flint is an epigenic product, consequent to an upheaval of Cretaceous calcium carbonate muds above sea-level, being deposited from surface solutions which contain silica from weathering processes, probably in Tertiary times.
So Goldschmidt appears to rule out radiolarians as the accepted origin of the silica and one can understand why he needs an "upheaval" of the chalk beds. But there is no mention yet of Eh/pH factors.
In 1960 Grabau is thinking about the idea that flints form around a nucleus of some skeleton and says there is no need for any nucleus but still favours an organic origin. Few seem to know Grabau despite the fact his writing is very good and his book appealing:
Chert concretions accept the same relation to limestone that flints do to chalk.
They are derived from the organic silica enclosed in the deposit, and redeposited in favourable places.
Confluent concretions of chert produce a more or less continuous chert bed such as is common in the Devonian limestones of eastern North America. Chert concretions may enclose organic remains, but they do not necessarily form around a visible nucleus. Van Hise holds that the heavier chert bands are formed first by the original segregation of siliceous organisms and that the subsequent enrichment by silica-bearing ground water of these siliceous strata.
It is true that these deposits have been regarded as formed directly in lagoons and cut-offs along the sea coast of the time. (He adds that this has been discarded by some workers.)
In 1967 Krauskopf describes the size of particles and even mentions pH. But there is still no mention of Eh or of the high sea-levels of the Cretaceous nor the epeiric seas. He introduces us to such terms as: sol, hydrophobic, hydrophilic. His writing is interesting and graphic:
Technically, colloidal particles may be either solid, liquid, or gas, and the dispersion medium likewise may be any one of the three states of matter. In geology we are concerned almost exclusively with water as a dispersion medium and with solids as the dispersed particles.
A few colloids are difficult to classify as either hydrophobic or hydrophilic. Silica, for example behaves like a hydrophilic colloid in that a dilute silica sol is stable indefinitely and in that it readily forms a gel. On the other hand, silica does not spontaneously disperse itself in water; in this respect it exhibits the characteristic behaviour of a hydrophobic colloid.
Colloid particles lie in the size range between that of true solution (10-7mm) and that of suspensions whose particles quickly settle out (>10-3mm). We can speculate that slight changes in pH and silica concentration might be responsible. A slightly acid solution supersaturated with silica could cause calcite to dissolve and silica to be deposited; a slightly alkaline solution undersaturated with silica could be responsible for the opposite change.
Krauskopf goes on to discuss pH as follows:
The pH of ocean water sampled near the surface is almost always between the narrow limits 8.1 and 8.3 although locally and temporarily it may deviate from this range, but by and large the pH stays surprisingly constant.
This is of interest because we know that silica is deposited within different limits of pH, namely between a pH of 7.0 and 7.8 as shown in Fig 1.
And finally it might be appropriate to close with what Twenhofel (1962) said about replacement:
The change often takes place atom by atom, so that the original structures are perfectly retained.
Bibliography
Goldschmidt, V.M. Geochemistry (1954)
Grabau, Amadeus W. Principles of Stratigraphy (1960)
Hatch, Rastall & Black Petrology of the Sedimentary Rocks (1936)
Holmes, Arthur Principles of Physical Geology (1944)
Krauskopf, Konrad Introduction to Geochemistry (1967)
Twenhofel, William Treatise on Sedimentation (1926) (Reprinted 1961)
Notes
1. The units used by Krauskopf in 1967 were not SI units which are recommended for all scientific work today. See article entitled: "What is Measurement?" in Humberside Geologist Number 8 (1991)
2. SOL: This word is not defined in the text. We can speak of a disperse phase in a disperse medium. The whole can then be called a "system". For example: smoke is a solid/gas system; fog is a liquid/gas system; emulsion is a liquid/liquid system.
An important type of sedimentary process is the solid/liquid system which includes sols, gels, and pastes. In these the liquid is water. Sols are systems that resemble liquids in terms of their physical properties. Sols flow like water but gels show rigidity.
Interesting research projects can be devised by every member of our Society. Even if you do not have any laboratory facilities you may conduct your own "thought experiments". Mull it over. And soon you will be devising some kind of apparatus to test out maybe just a single part of the suggested ideas listed below:
1) How might it be possible to set up a simple experiment to test the pH at the sea water/ sediment interface? Note that it-is accepted that sea water has a pH within the narrow limits of 8.3 to 8.6 so one is checking to see if the pH changes to 7.8 at the interface.
2) How might it be possible to check in a simple experiment the depth below the sea water/sediment interface where the pH becomes suitable for the deposition of flint?
3) Assuming the suitable pH is below the sea water/ sediment interface, what sort of apparatus would you devise to check the difference between vertical and horizontal flow of pore waters? Does this affect shape?
4) If we assume that flint is deposited in a range of pH 7.0 to 7.8, how might you determine the width of the band of suitable pH within the sediment?
In conducting your experiments would you obtain some chalk and powder it and compact it to resemble an early environment of deposition. Would this provide an appropriate pore space? Or would you obtain solid chalk? Might spaces around the edge of the solid or cracks within it affect your experiment? If so, how would you deal with this problem?
Why not write up your ideas, complete with simple sketches of the suggested apparatus you devise, and send them to the Editor of Humberside Geologist ?
copyright Hull Geological Society 2021