
Humberside Geologist No. 14

Humberside Geologist No. 14
The Geochemistry of the Red Clays of Holderness and their origin.
Extra data is available on the CD-ROM of Humberside Geologist number 14.
Abstract.
This research was carried out as a forensic science undergraduate project at the University of Hull, Department of Chemistry. Samples of red clay (with no gravel or pebble content) and red boulder clay from the coast of Holderness were compared with clays of known geological age and origin. The techniques used included ICP analysis, SEM analysis, acid carbonate testing, microscopy and particle sizing. The red boulder clays showed similarities with a weathered boulder clay. The red clays showed similarities with unweathered boulder clay. They showed no affinity to the Red Chalk or Mercia Mudstone.
Introduction
This project was trying to ascertain if it was possible to prove conclusively whether one or more rocks came from the same source by using currently existing analytical methods and instruments. To answer this question several different samples of red clays were selected for use in this experiment.
Geological background
The Boulder Clays (or Tills) of Holderness were deposited by the Ice Ages of the late Quaternary by glaciers that had travelled from the Lake District, Scotland, Northern England and Scandinavia. We have evidence for this from the erratic rocks and fossils that can now be found in Holderness (Horne & Harrison 1992, Horne 2000, Rockett 1992).
Thin layers of red and grey clay and soft chalk can be seen between the layers of the Tills in the cliffs of Holderness or occasionally as beach exposures (Bisat in Catt and Madgett 1981, Fenton 1977). The chalk in these 'rafts' is younger that the chalk exposed in East Yorkshire, and has probably been picked up by the glaciers as they moved across the bed of the North Sea (Horne, ongoing research). The grey clays seem to be of Late Jurassic age, from evidence collected by Stuart Jones and Chris Brogden. But what is the origin of the bands of red clay and red boulder clay? There are several possibilities :-
Samples of red clay from a beach exposure at Aldbrough,
East Yorkshire [sample
numbers ALD2005-3, ALD2005-5, ALD2005-VR] and Red Boulder Clay from the
"Red Band" in the boulder clay cliffs at Aldbrough
[ALD2005-2] , Hornsea [HORNRBC] and Mappleton
[MAP2005] were collected by Anne Horne, Mike Horne and Gordon Ostler
in 2005. These were compared with other samples of red clays and boulder
clay.
|
|
|
|
|
| ALD-2005-2 | Aldbrough (NGR TA260392 ) | Pleistocene | Red boulder clay |
| ALD-2005-3 | Aldbrough(NGR TA260392 ) | Pleistocene | Red clay |
| ALD-2005-5 | Aldbrough(NGR TA260392 ) | Pleistocene | Red laminated clay |
| ALD-2005-VR | Aldbrough(NGR TA260392 ) | Pleistocene | Very red clay |
| BOOKBARN | Book Barn, Hallowtrow, Somerset. (NGR ST6356 ) | Triassic | Red clay - Mercia Mudstone Group |
| HOLMEOSM | Church Hill, Holme-On-Spalding-Moor. (NGR SE819388) | Holocene | Red Soil - ?derived from Mercia Mudstone Formation or an early glaciation. |
| HORNBC | Hornsea (NGR TA2118446941 ) | Pleistocene | Boulder Clay (unweathered) |
| HORNRBC | Hornsea(NGR TA2118446941 ) | Pleistocene | Red Boulder Clay |
| HORNWBC | Hornsea(NGR TA2127746629 ) | Quaternary | Weathered Boulder Clay |
| MAP2005 | Mappleton(NGR TA230434 ) | Pleistocene | Red boulder clay |
| SE452766 | Sugar Loaf Bay, Portishead, Somerset. (NGR SE452766) | Devonian | Red clay from the Old Red Sandstone. |
| SPEETON | Speeton (NGR TA153755 ) | Albian, Cretaceous | Red marl from Red Chalk Formation. |
Most of the samples were collected from coastal locations, except SE452766, HOLMEOSM and BOOKBARN.
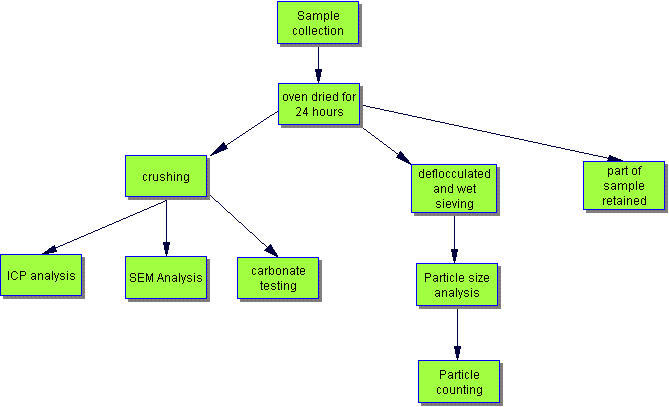
Techniques used -
[safety note - the specialist chemical techniques were carried out in a science laboratory and for safety reasons should not be repeated at home!]
ICP
Inductively Coupled Plasma- Optical Emission Spectrometry (ICP-OES) is a solution based technique for elemental analysis. An ICP is a very high temperature (7000-8000 K) excitation source that efficiently desolvates, vaporises, excites and ionises atoms.
The most essential step in analysis is the sample preparation; as unless appropriate sampling schemes and suitable procedures are used to prepare samples any subsequent analysis will be wasted. The sample needs to be ground up to give a small particle size and then dissolved in acid. A weak acid is generally referred to as a leach and while it may result in a quantitative extraction of the elements of interest, most of the sample will remain undissolved. Strong acid attacks are generally referred to as digestions and these are more powerful than leaches. For the experiment carried out in this project, aqua regia digestion was used. Aqua regia is a 3:1 mixture of hydrochloric and nitric acids. Nitric acid destroys organic matter and oxidises sulphide material. It reacts with concentrated hydrochloric acid to generate aqua regia according to the equation:-
3HCl + HNO3 ==> 2H2O + NOCl + Cl2
Aqua regia is an effective solvent for most base metal sulphates, sulphides, oxides and carbonates but only provides a partial digestion for most rock forming elements. This acid digestion is greatly speeded up when combined with microwave digestion. This prepares samples at high temperature in closed vessels and allows smaller quantities of acid to be used. It also ensures that certain inorganic compounds that are quite resistant to acid attack are digested. The digested sample can then finally be diluted and analysed. This analysis could then give detailed information on the chemical composition of the samples.
An aqua regia solution (100 ml) was made by mixing concentrated hydrochloric acid (75 ml) and concentrated nitric acid (25 ml). The solution was left to stand overnight in a fume cupboard to allow the chlorine gas to escape and the bright orange colour to develop. Each sample (0.3 g) was added to the digestion containers and aqua regia solution (5ml) added before the containers were loaded into the microwave digestion machine. This digestion was carried out at 80 p.s.i. for 10 minutes and then the samples left to cool. The samples were removed and added to a 25 ml conical flask ensuring thorough rinsing of the containers and then the flasks made up to the mark with water. These samples were diluted further by taking 1ml of sample and adding it to a 10 ml conical flask and made up to the mark with 2% HNO3. The samples could then finally be analysed using the ICP-OES instrument.
Scanning Electron Microscopy
SEM analysis involves directing a beam of electrons at the sample. Electrons are emitted from the sample and collected by a detector which converts them into a small electric signal. This signal contains a variety of information about a single point on the sample surface. To form an image of the sample a large number of points over an area need to be analysed, and the final image is built up from the number of electrons emitted from each point. To enable all of this to occur, samples need to be prepared carefully to withstand the vacuum inside the microscope but they also need to be made conductive. This is achieved by sputtering or vacuum evaporation to put a thin carbon or metallic coating onto the sample.
Scanning electron microscopes are often coupled with x-ray analysers. This is because x-rays are produced whenever an electron beam interacts with matter and these can be used very effectively to give information about the chemical compositions of the sample. Energy Dispersive X-ray micro analysers (EDX) are the most commonly used as these can detect almost all elements simultaneously and these elements are identified from their characteristic energies and their concentration can be derived from the count rate. This chemical composition is achieved using bulk analysis rather than spot analysis. This is because bulk analysis gives an area analysis and so the best overview of the sample, whereas spot analysis is best used for analysing a specific grain.
The sample pellets were produced by the method and equipment normally used to make K Br discs for Infra-red analysis. The powdered samples and pellets were then sent for analysis. The analyses represent an average of five spot samples.
Acid Carbonate Testing
A recorded amount (about 25g) of each of the powdered rock samples was taken and mixed with a small amount of de-ionised water and then dilute hydrochloric slowly was added. Once the samples had stopped fizzing more acid was added until the reaction with the samples was complete. The samples were filtered to remove the liquid and the remaining solids transferred to filter paper and dried. After thorough drying the samples could be re-weighed and the carbonate content calculated. The acid reacts with the calcium carbonate in the rock samples and hence the weight of the rocks, once the acid has been filtered off, decreases providing an indication of the amount of carbonate in each of the samples.
Particle Sizing
For this analysis the samples were first boiled in water with a de-flocculating agent to break the rock down into a slurry. This slurry was put through a set of sieves to filter out the minerals and bits of rock in the sample, and this allowed all the silt and clay to be removed. The set of sieves used had the following mesh sizes: 63um, 250um, 500um and 1mm.
Once the particle sizing had been carried out microscopic analysis of the fractions could be done to see what grains, and possibly minerals were present. This part of the research used the particle-sized samples: a selection of the samples from the 500 um -1mm and 250 um -500 um particle size ranges were looked at under a microscope. The different types of particulates seen were categorised and counted. About 200-300 particulates were counted for each sample; this was to ensure that the results obtained gave a true representation of what each sample contained.
Results and discussion -
ICP analysis -
ICP analysis provided the best results of the project in terms of answering the question of whether the samples could be matched to one another. Although it could not provide a definite answer that two samples were identical, it did show there were two groups of similar compositions, and one group with significantly different compositions to the rest.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Group A
BOOKBARN, SPEETON, SE452766 and HOLMEOSM.
BOOKBARN and SPEETON are very obviously different to the other samples. BOOKBARN contains a very large amount of iron whilst SPEETON is mostly made up of calcium. SE452766 and HOLMEOSM have elemental compositions that are very similar but are from opposite ends of the country, with SE452766 originating from Somerset and HOLMEOSM from Yorkshire. Therefore these two samples are unlikely to have originated from the same source even though they are elementally similar.
Group B
HORNBC, ALD-2005-5, ALD-2005-3 and ALD-2005-VR.
All of these samples have virtually the same elemental composition. They contain about 5% calcium and have a significant amount of iron in them. HORNBC was essentially used as a standard as it was known to be a boulder clay. Therefore the conclusion can be drawn that the other three samples in the group are most probably also boulder clays and originate from the same area/source.
Group C
HORNWBC, ALD-2005-2, MAP2005and HORNRBC.
These four rocks are compositionally similar; they contain roughly an equal ratio of iron and calcium. HORNWBC is known to be a weathered boulder clay and in comparison to HORNBC (boulder clay) has less calcium but more iron present. This could show that the weathering slightly alters the percentage elemental composition of rocks. Hence, HORNRBC, ALD-2005-2 and MAP2005could originate from the same area/source as HORNWBC or it could just be rocks that have undergone a similar weathering process.
Although these differences cannot be explained in terms of locations, it does provide evidence that samples have similarities in their various compositions. It may also be an indicator that they possibly originate from the same areas before the glacial movements of the Ice Age.
SEM Analysis -
Morphological information
Scanning Electron Microscopy (SEM) provided images of the powdered samples at a micrometre size, as well as a basic elemental composition of the sample. Pictures of all 12 of the samples were taken using the scanning electron microscope; each sample was photographed at three different magnifications.
The morphological information is seen the SEM pictures. These were taken at three different magnifications for each sample. To obtain the pictures a smooth surface needs to be achieved which is done by grinding the sample into a fine powder. Unfortunately this destroys some of the surface characteristics and so an accurate comparison of the samples cannot be carried out. It is also difficult to carry out a comparison due to the samples all being quite similar and there being no characteristic features such as fossils being present.
Compositional information
Using an X-ray detector, an elemental breakdown on the samples was also possible using the SEM apparatus. The compositional data was obtained by randomly analysing five spots on the sample and then taking an average of the results. The results of this are summarised in the tables below:
Raw Data
It can be seen that all of the samples had very similar silicon (approx
25%) and oxygen (approx 45%) content, but once these elements were removed
the charts started to show a large correlation to the ICP results. From
the SEM compositions the samples can be grouped in the same way. Group
A are the samples that are very different to the others and it can be seen
again that the SPEETON sample has a very
large calcium content, whilst BOOKBARN has very little calcium but large
amounts of iron. The weathering effect on samples can also be seen with
these results. The calcium content decreases between group B and C, but
an increase in the iron content was not seen with the SEM results as was
seen from ICP.
Carbonate testing
% carbonate = ((original weight - weight after digestion) /original
weight) x 100
The acid test showed that all of the samples contained some carbonate
but in varying amounts. This was expected due to all of the samples being
soils and so most probably containing some quantity of chalk.
SPEETON contained a very large amount of carbonate; this quantity was
much larger than that in any of the other samples. By contrast, BOOKBARN
contained very little. It contained only 10% of the quantity in the SPEETON
sample. These results therefore correlate with those found from the ICP
analysis.
Particle sizing
Particle sizing was carried out for seven of the samples and the following
results obtained:-
Another surprise was that HORNRBC showed the smallest amount of particles
under 63 um, as this was assumed to be
the case for HORNWBC – the weathered sample. An explanation however could
be correlated with the colour of the sample. As ICP analysis found, the
majority of the samples had a large percentage of iron in their composition,
and the oxidation states of iron give red colours i.e. rust. A possible
explanation therefore is that this sample had a degree of oxidation to
it. This may have been due to water or weathering whilst it was still in
formation during the Ice Age, and consequently some of the material may
have broken down.
The fractions were placed into three groups according to their amounts
within the 250 -500 um range. These were:
a) BOOKBARN, MAP2005, and HORNWBC
b) HORNRBC and HORNBC
c) HORNVRC and SPEETON.
Unfortunately, these fraction percentages appeared to be the only similarities
between the samples, as when comparing these groups for the other fraction
similarities there are none.
Microscopy
Once the particle sizing had been carried out microscopic analysis of
the fractions could be done to see what grains, and possibly minerals were
present. The particle size 250 - 500µm was mainly studied and the
following results obtained:-
The results form the optical microscopy
are very varied from sample to sample, and again no patterns can be spotted
between the samples analysed. This may again be due to the limited number of
samples analysed in this matter, as only half of the 12 samples were used, and
so the spectrum of samples was not as big as for the other methods.
Another big problem is the classification system employed in this
procedure. As none of the people that undertook this technique had much geological
knowledge the classification system used relied mainly upon colour, size, shape
and lustre. This means that the analysts used some personal discretion. For
example what one person may believe is a smoothed black particle, could to another
scientist be seen as a rounded dark grey particle. Basically, due to the specialist
nature of this technique it could not be performed to its full potential.
The results that were found were placed into three groups based
on the percentage of ‘clear quartz’ counted within the samples. This method
would have been more beneficial to the project if it had been carried out by
a geologist. It was found that although not exact, the two different sizes of
HORNBC did have a rough similarity, which was expected considering the sample
itself did not change in any way. It was interesting that based on this grouping
there was some degree of matching possible; as the results show the BOOKBARN
and SPEETON samples are grouped together for the ICP results and appear to have
a similar number of clear quartz crystals; and MAP2005and HORNWBC also follow
this trend. All four however from this analysis do have similar particle compositions
though, hence the findings were considered inconclusive.
The three groups were:
a) HORNBC at 250 and 500 um
b) ALD-2005-VRC 250 um and HORNRBC
500 um
c) BOOKBARN and SPEETON at 500 um.
Conclusions
The SEM analysis has managed to produce some results extremely
similar to those produced by the ICP. As an example of how well the results
seem to compliment each other, the results for the amount of calcium found in
the samples by ICP and SEM are compared below with the carbonate content:-
So, the SEM results and ICP results appear to agree on the elemental
compositions for most of the elements.
The acid digestion results also seem to be correct when used in
conjunction with the ICP and SEM data collected on the amount of calcium in
the samples, as can be seen in the table to the left as well. There is clearly
a correlation between the percentages in all three of the techniques; they all
increase with respect to each other in most cases. It does appear that the SEM-EDX
may not be as sensitive as the ICP and the acid digestion calculation is slightly
off for some of the samples. This could be due to the acid dissolving more than
just the calcium in the sample, and so the calculated values may not be completely
accurate.
The samples analysed seem to fall into three groups; in two of
the groups the samples are very similar chemically -
A - A diverse group that show no particular similarities to the
other groups. The Holme-on-Spalding-Moor sample
[HOLMEOSM] is a clay soil from the Vale of York: could the high magnesium level
be from an influence from Magnesian Limestone
in a glacial deposit, or is the higher potassium and magnesium from agricultural
fertilisers? The marl from the Speeton Red
Chalk [SPEETON] has a high calcium carbonate
content. Previous studies have revealed no microfossils of Albian
age (Horne unpublished) in the Red Boulder Clay bands. The Mercia Mudstone [BOOKBARN]
showed high iron content and low calcium carbonate.
B - ALD-2005-3, ALD-2005-5, ALD-2005-VR and HORNBC. The three
red clays from the beach at Aldbrough have
a similar chemical composition, as well as similar grain size distributions.
They are similar to the chemistry of the un-weathered boulder clay from Hornsea.
Could they have been washed out of an unweathered
Boulder Clay? There are also some similarities with the Old Red Sandstone sample
from Somerset which are hard to explain.
C - ALD-2005-2, HORNRBC, MAP2005 and HORNWBC. The three red boulder
clay samples were collected from sites several kilometres apart and show great
similarities with the weathered Boulder Clay. They are from the Red Band between
two Boulder Clays and could therefore represent a layer of weathering or a smear
of weathered boulder clay pushed along by the glacier.
Overall though the data collected from the three more chemical
processes it appears that many of the results seem to support the three groups
theory. The physical processes employed turned out to be a little disappointing
especially the microscopy, as it was hoped that a comparison between the SEM
pictures and what could be seen down an optical light microscope could be compared
but unfortunately this was not possible. The particle sizing also gave no relevant
information.
Acknowledgements -
Thanks to the project supervisors Tony Walmsley
and Mike Horne, Tony Sinclair for his help with the SEM analysis, Bob Knight
for his help in the ICP analysis, Marion Brazier and Mark Anderson of the Geography
Department for the loan of equipment and the Chemistry Department technicians
in the laboratory for their helpfulness throughout this project. The project
was funded by the University of Hull Department of
Chemistry.
References and bibliography -
Catt J A & Madgett P A
1981. The work of W S Bisat
F.R.S. on the Yorkshire
coast. pp 119-136 of Neale & Flenley The
Quaternary in Britain. Pergamon Press. 267
pp.
Fenton K 1977. Bridlington to Hornsea.
Humberside Geologist. 2, 10 (not numbered).
Hill S J (ed.) 1998. Inductively
coupled plasma spectrometry and its applications.
Horne M & R Harrison
1992. The East Riding Boulder Committee, reports for the years 1987 to
1991. Humberside Geologist 10,18-22.
Horne M 2000. Report of the East Riding Boulder
Committee 1992 to 2000. Humberside Geologist 13, 42-45.
Horne M, Rockett
T & Whitham F 2000. The Glacial
Geology of Dimlington High Cliff Humberside
Geologist 13, 53-56.
Horne M 2005. Hull Geological
Society’s standard methods for recording Quaternary sediments (proposals).
<http://www.horne28.freeserve.co.uk/flamsop.htm>
Lawes
G 1987 Scanning electron microscopy and X-ray microanalysis. John
Wiley & Sons.
Perrin M B 1975. An
Introduction to the Chemistry of Rocks and Minerals. Edward Arnold.
Pye K & D J Croft (eds.) 2004.
Forensic geoscience: principles, techniques
and applications Geological Society [London] Special Publication
no. 232. 318pp
Reeves R.D.& R.R. Brooks 1978. Trace
Element Analysis of Geographic Materials. John
Wiley and Sons.
Rockett T 1992. Glaciation
and the YorkshireCoast. Humberside
Geologist 10, 14.
Smith K A 1983. Soil Analysis:
Instrumental Techniques and Related Procedures. Marcel Dekker Inc.
Weaver C E 1989. Clays, Muds,
and Shales Elsevier.
Winefordner J D (ed)
1996 Introduction to x-ray powder diffractometry. John
Wiley & Sons.
copyright Hull Geological
Society 2020
Percentages without oxygen and silicon:-
SPEETON
SE452766
MAP2005
HORNWBC
HORNRBC
HOLMEOSM
BOOKBARN
ALD-2005-VR
ALD-2005-5
ALD-2005-3
ALD-2005-2
HORNBC
Notes - All samples were collected from the beach or low cliffs at coastal
exposures, except HOLMEOSM and BOOKBARN which are inland, and HORNWBC which
was from the top of a cliff section: the chlorine levels probably reflect
the influence of sea water.
SPEETON
SE452766
MAP2005
HORNWBC
HORNRBC
HOLMEOSM
BOOKBARN
ALD-2005-VR
ALD-2005-5
ALD-2005-3
ALD-2005-2
HORNBC
Sample
Dried
weight (g)
Weight
after acid digestion (g)
%
carbonate
HORNBC
HORNWBC
MAP2005
ALD-2005-3
ALD-2005-2
ALD-2005-VR
HORNRBC
SPEETON
BOOKBARN
ALD-2005-5
SE452766
HOLMEOSM
%
%
%
%
%
SPEETON
BOOKBARN
ALD-2005-
VRC
HORNBC
HORNWBC
HORNRBC
MAP2005
HORNBC,
250 um
amount
percent
clear
quartz
yellow
quartz
white
quartz
grey
black
orange
dark
grey
TOTAL
ALD-2005-VR,
250 um
amount
percent
clear
crystal quartz
dull
crystal quartz
large
multicoloured rough quartz
grey
sparkly angular
black
(shiny silver)
black
rough irregular
angular
orange quartz
red-brown
rough
white
(chalk)
TOTAL
HORNBC,
500 um
amount
percent
clear
quartz
yellow
round/ orange
white
round quartz
brown
round
black
angular sparkly
quartz
other
TOTAL
BOOKBARN
500 um
amount
percent
clear
angular quartz
off
white angular quartz
grey
shiny round
black
angular
red
brown rough irregular
TOTAL
HORNWBC
500 um
amount
percent
clear
smooth angular quartz
unclear
rough irregular quartz
grey
smooth angular
black
smooth angular
black
rough rounded
orange
rough irregular
smooth
brown angular quartz
white
rough rounded
red
sparkly irregular
TOTAL
(c) Hull Geological Society 1999 + 2007